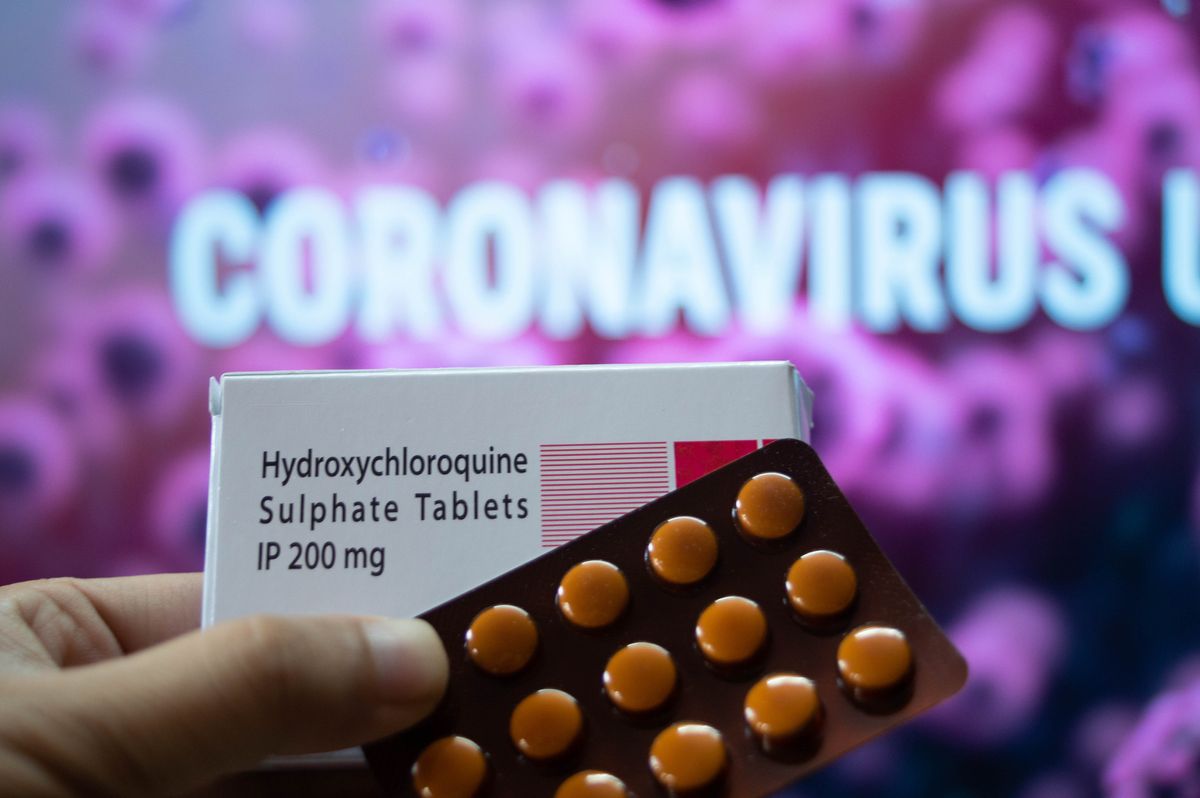
FDA revokes emergency authorization of hydroxychloroquine for COVID-19 treatment
A few minutes every morning is all you need.
Stay up to date on the world's Headlines and Human Stories. It's fun, it's factual, it's fluff-free.
On Monday the United States Food and Drug Administration (FDA) rescinded its emergency authorization of anti-malarial drug hydroxychloroquine, the drug that has been repeatedly endorsed by President Donald Trump as an effective COVID-19 treatment.
“FDA has concluded that, based on this new information and other information discussed in the attached memorandum, it is no longer reasonable to believe that oral formulations of HCQ and CQ may be effective in treating COVID-19, nor is it reasonable to believe that the known and potential benefits of these products outweigh their known and potential risks," FDA Chief Scientist Denise Hinton wrote in a letter to Gary Disbrow, the acting president of the Biomedical Advanced Research and Development Authority (BARDA) on Monday.
On the same day, the FDA also warned against the administration of the drug to patients taking remdesivir, an antiviral drug by Gilead Sciences Inc. that has received authorization for treatment of COVID-19. The statement explained that the combination of the two drugs could reduce the effectiveness of remdesivir.
“The agency is not aware of instances of this reduced activity occurring in the clinical setting but is continuing to evaluate all data related to remdesivir," the FDA said.
The FDA’s recent decision will not affect clinical trials of hydroxychloroquine.
Hydroxychloroquine first gained attention in February when small and non-peer reviewed reports from China suggested that it could be a potential treatment for COVID-19. Prior to this report, it was the 128th most commonly prescribed medication in the US, with more than five million prescriptions in 2017.
The FDA issued authorization for the drug on March 28 to treat patients hospitalized with COVID-19 and on April 13, Trump announced that 29 million doses of the drug had been deployed by his administration.
However, on April 24, the FDA warned doctors against prescribing the drug to patients outside of clinical trials, citing reports of “serious heart rhythm problems” in patients who had taken the drug.
Despite the FDA’s warnings, Trump continued to promote the drug and in mid-May, claimed that he had been taking hydroxychloroquine as a preventive measure after two of his aides had fallen ill with the virus.
The FDA’s announcement also comes amid recent studies that have shown hydroxychloroquine to be ineffective in treating COVID-19.
A study conducted in the United Kingdom early this month found no evidence of the drug’s ability to treat COVID-19 patients. Another trial conducted by the University of Minnesota also concluded that the drug did not prevent healthy individuals from contracting the virus after coming in contact with a COVID-19 positive patient.
Hydroxychloroquine will continue to be prescribed to patients for malaria, lupus and rheumatoid arthritis – illnesses for which the drug has approval.
Have a tip or story? Get in touch with our reporters at tips@themilsource.com




Comments ()