Three coronavirus vaccine trials produce immune response in humans
A few minutes every morning is all you need.
Stay up to date on the world's Headlines and Human Stories. It's fun, it's factual, it's fluff-free.
As countries race to develop a vaccine for the coronavirus, three laboratories released findings on Monday that show promising results. The studies – published by developers from the United Kingdom, China and a joint venture between the United States and Germany – all reported that early trials of their vaccines had produced immune responses in humans.
Two of the vaccine developers published their findings as peer-reviewed studies in the respected British medical journal The Lancet.
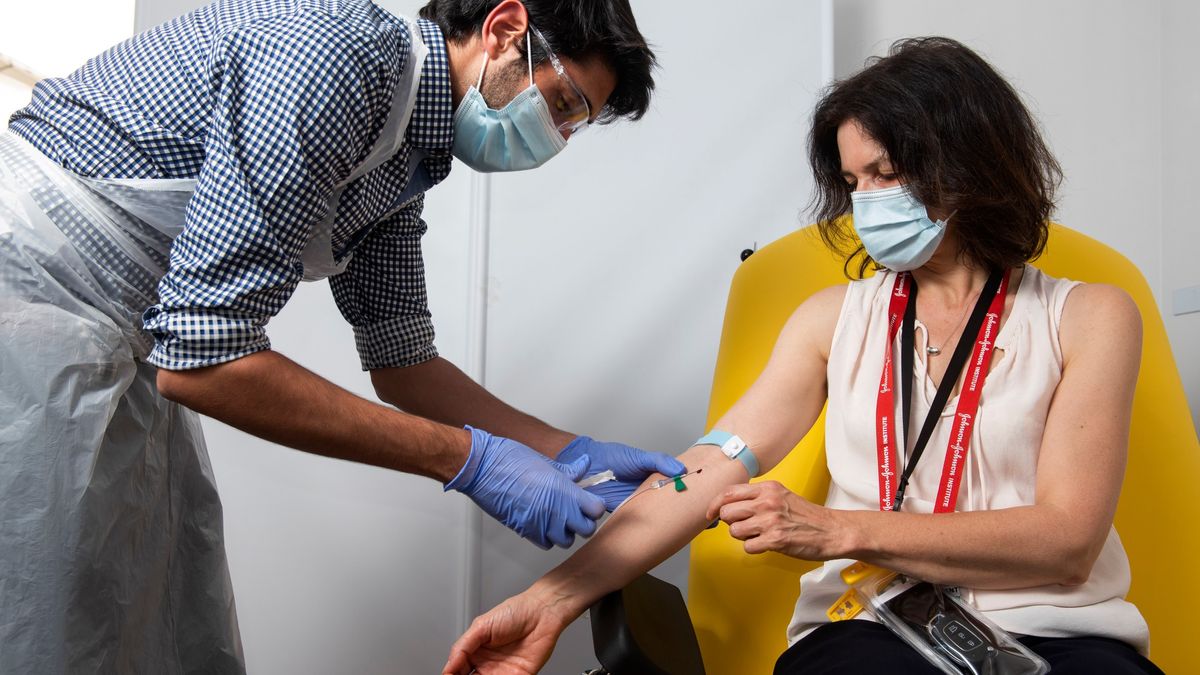
The first was a partnership between the University of Oxford and the British-Swedish pharmaceutical company AstraZeneca. It is also believed to be one of the most advanced and promising vaccine efforts currently in development and is the first vaccine to begin Phase III trials in large-scale tests.
The treatment has already been administered to over 10,000 participants from the UK, Brazil and South Africa. Another large-scale test is due to begin next week involving an expected 30,000 participants in the US.
The Oxford vaccine, which has been in development since April, has been found to induce neutralizing antibodies similar to those found in COVID-19 survivors in 91% of participants a month after receiving one dose and in 100% of participants who received a second dose. However, 70% of participants experienced common side effects, such as fatigue and headache, but none were found to be too serious.
70% of participants from the Oxford vaccine reported experiencing fatigue and 68% reported experiencing headaches. In total, only minor side effects were reported in this study.
The second study published in The Lancet was conducted by the Chinese vaccine development company CanSino Biologics, which used a similar technique to the Oxford study. It, too, prompted an immune response in participants.
“Single-dose immunisation with the vaccine induced rapid onset of immune responses within 14 days, and significant humoral and cellular immune responses within 28 days in the majority of the recipients,” said CanSino scientists.
77% of participants also reported experiencing negative side effects such as fever or pain at the injection site after being vaccinated, but none reported serious adverse reactions.
However, their preliminary findings showed variation in the vaccine’s efficacy among individuals who had different levels of immunity or previous exposure to a virus used in the vaccine. Researchers also noted that their participants – all from Wuhan, China – were not exposed to the coronavirus after receiving the vaccine, so it is still unclear if it will prevent infection.
Despite the fact that the vaccine’s efficacy has yet to be tested in a large-scale study, Beijing has already authorized the military to use the CanSino vaccine.
“Overall, the results of both trials are broadly similar and promising,” said Naor Bar-Zeev and William Moss, two vaccine experts from the John Hopkins Bloomberg School of Public Health.
A joint development effort between the German biotech company BioNTech and the US pharmaceutical giant, Pfizer, also released promising findings on Monday. This comes on the heels of a similar vaccine trial from American company Moderna Inc., which released its own early findings last week.
Their trial was done on a smaller scale and was based on 60 participants in Germany.
While it is still uncertain whether or not any of these treatments provide long term protection from the coronavirus or could potentially put an end to the pandemic, the findings are nevertheless a glimpse of hope during dark times.
Governments and other entities are already lining up to support vaccine efforts. The US, UK and several others have already committed to investing hundreds of millions of dollars in the Oxford study. This would contribute to the production of up to two billion doses and also establish a guaranteed supply after production.
The UK has also struck a deal to secure 30 million doses of the vaccine developed by BioNtech and Pfizer.
“It’s encouraging that all these vaccines seem to induce antibodies in people,” said Marie-Paule Kieny, the former World Health Organization (WHO) assistant director-general. “This proves that the science is moving forward very quickly, which is a good sign.”
Some also argue that there is a place for all three of these vaccines in order to effectively treat the global population given the different needs of people from varying demographic groups.
However, experts remain cautiously optimistic about the findings and urged the public to recognize the uncertainty of the results.
“What this means is that each of these vaccines is worth taking all the way through to a Phase III study,” said Dr. Peter Jay Hotez, a vaccine researcher at the Baylor College of Medicine. “That is it. All it means is ‘worth pursuing’,” he added.
“All the hype makes it seem like a miracle is around the corner and that is just not the case,” said Hotez.
“This is not going to be a quick fix. This is going to take years to sort out.”
Have a tip or story? Get in touch with our reporters at tips@themilsource.com




Comments ()